End-to-End Support of Studies involving DXA:
Obesity, Osteoporosis, Oncology, Infection disease, Nutrition and Metabolism Studies
Streamlined DXA Analysis for Clinical Trials
Did you miss our live discussion?
Watch this webinar here.
MAKE OUR EXPERT TEAM YOUR NEW PARTNERS
Unparalleled Imaging Expertise: With over 20 years of experience in DXA, MRI, Fibroscan, and X-ray for clinical trials, IAG excels in both large registrational studies and early-phase projects.
Award-Winning Data Management Platform: IAG’s DYNAMIKA platform efficiently processes imaging data, supports all trial stakeholders, and delivers results via reports or dashboards, enabling rapid decision-making.
Time-Sensitive Solutions: Understanding the critical nature of time in clinical development, IAG provides transparent, efficient support to partners, readers, and sponsors throughout the trial process.
Global Support, Local Focus: We placed our team of imaging specialists in geographically important hubs (USA, EU, Asia, UK), which enables them to provide efficient answers to the sites and support global trials, with speed.
Value-Driven Partnerships: We prioritize delivering the highest quality data at competitive cost. We thrive to build long term relationships with our partners and clients.
QUALITY & EFFICIENCY
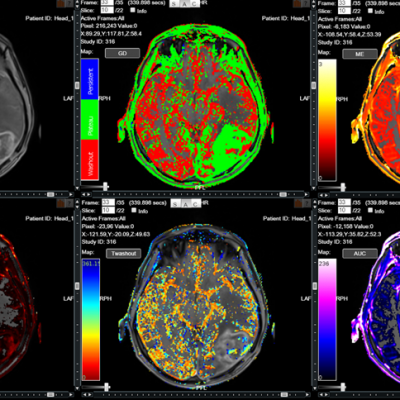
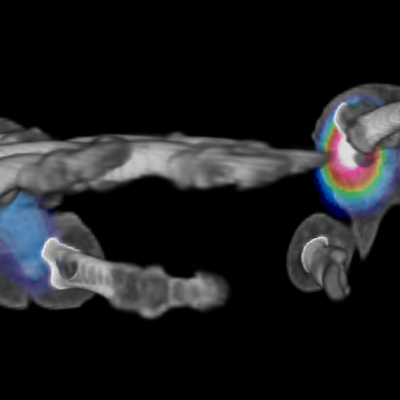
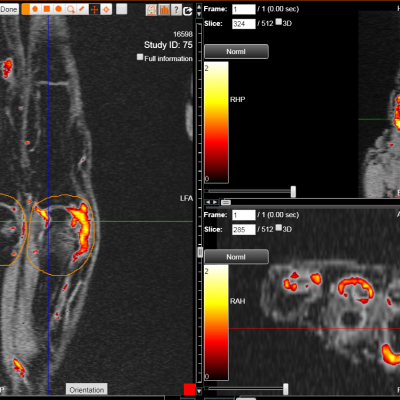
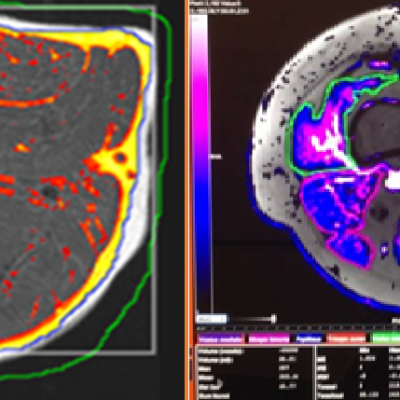
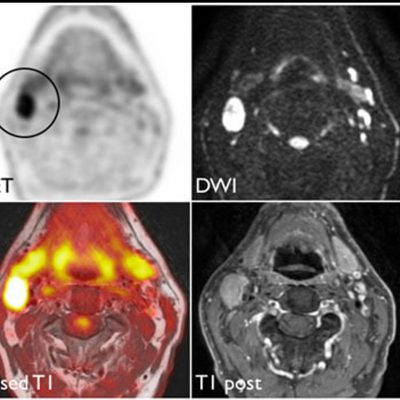
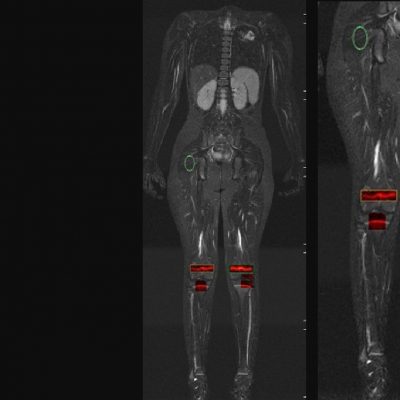
We specialize in building imaging-powered differentiation strategies for novel drug candidates and biosimilars, with a strong focus on safety and efficacy trials in bone health, obesity, osteoporosis, sarcopenia, and related areas. IAG’s team experience with using DXA, MRI, Fibroscan and X-ray in clinical trials spans over 20 years, with the company supporting large registrational studies and early phase projects. IAG’s proficiency with DXA imaging allows for precise measurement of lean mass, fat mass, and bone density changes, which are crucial metrics in evaluating the efficacy of obesity drugs.
Discover how IAG can drive significant cost savings (up to 35% trial budget reduction) and improve your chances of success through advanced patient stratification strategies that reduce trial length and cost. Partner with IAG to accelerate your path to market and maximize the impact of your groundbreaking therapies.
Case Study: One specific study, involving DXA analysis makes a vivid example. In 2021, IAG was contacted by a biotech company to support their Phase III trial. The objective was to utilize X-ray and DXA scans for combined eligibility as one of the criteria for the inclusion / exclusion of patients in their protocol. The Sponsor was looking for experts in the field, who can bring global network of sites, and execute screening with speed. IAG’s team partnered with the CRO and together we embarked on accelerating recruitment, opening out network of 750 obesity focused sites. The Study recruited faster than exepcted, saving Sponsor significant time and money. The data is currently being finalized to be submitted for regulatory review.
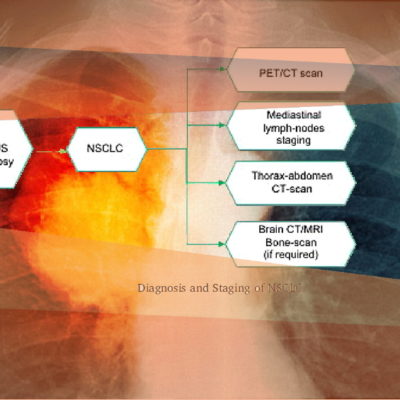
Given market dynamics, our latest focus has been on the safety and efficacy trials targeting bone health, obesity, OP, advanced oncology, sarcopenia, IO, spinal fusion.
Read how we can save costs: ‘35% Trial Budget Reduction’ and improve chances of success ‘Reducing Length & Cost of Trials through Patient Stratification‘.
+1200 patients
+50 Sites in all Europe
0.4 day from the upload to the QC report
2.2 days from QC report to Final Report
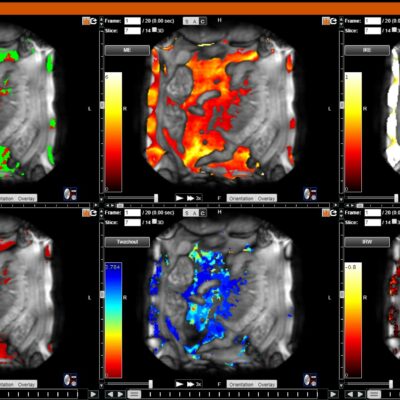
DYNAMIKA
hosted all the DXA and X-ray images and analysis
KEY TO DXA STUDY SUCCESS
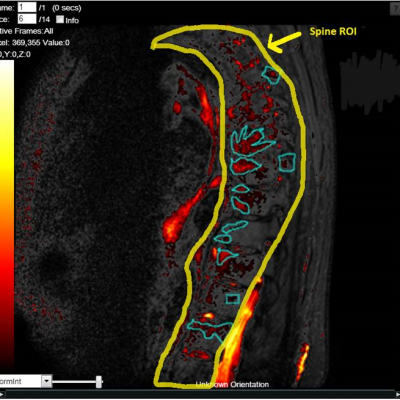
Reproducibility: Properly acquired and correctly analysed DXA scans result in very low precision errors, allowing the detection of subtle changes over time.
Validity: Evaluating and monitoring instrument calibration throughout your study is vital for validating patient BMD results. IAG ensures that Instrument Quality Control (IQC) data are collected correctly and available for analysis to support patient results.
Accessibility: IAG has collaborated with numerous imaging facilities and can recommend high-performing DXA sites for your study, reducing risk and improving patient recruitment.
Transparency: DYNAMIKA is a proprietary, secure image transfer and processing system that provides real-time access to tracking information throughout the study.
Would you be interested in receiving regular updates and
staying informed with the latest news?
We invite you to subscribe to our newsletter.
MULTIPLE SITES & ONE STUDY: SIMPLIFY THE DATA MANAGEMENT

Managing data across multiple sites in clinical trials can be complex and time-consuming. Recognizing this challenge, our team of experts developed DYNAMIKA. It is IAG’s proprietary enterprise-scale cloud-based platform for imaging processing and data management. It works across multi-centre trial settings to enable central imaging review. This one comprehensive software system controls trial progress, conducts central reads and, when needed, it can be integrated with AI tools for earlier read-outs and decision support. Read more here.
MORE THAN A PARTNER, AN EXTENSION OF YOUR TEAM
Image Analysis Group is a unique partner to life sciences companies. Our goal is to accelerate novel drug developments, by using the right analytical tools and a modern trial infrastructure. At IAG, we are committed to helping our biotech and pharma partners to efficiently develop novel life-changing medicines, accelerating their R&D pipelines through advanced analytics, IAG’s technology solutions and imaging contract research services. We see each project as an oportunity to build a long lasting partnership and to become an extension of your team.